Developed with input from research professionals, Clinasyst directly addresses the unique needs of research sites.
Quickly and efficiently perform study feasibility and identify potential study patients.

Accounts payables, account receivables, and invoiceables. Never miss getting paid for a completed procedure again!
Track and optimize your advertising campaigns.
Assign tasks to specific employees and follow through to completion to maximize your productivity and efficiency.

Simplify workflows, update CRFs in real time, instantly share updates with your team, and submit SAE reports, invoices, and patient referrals at the speed of sound.
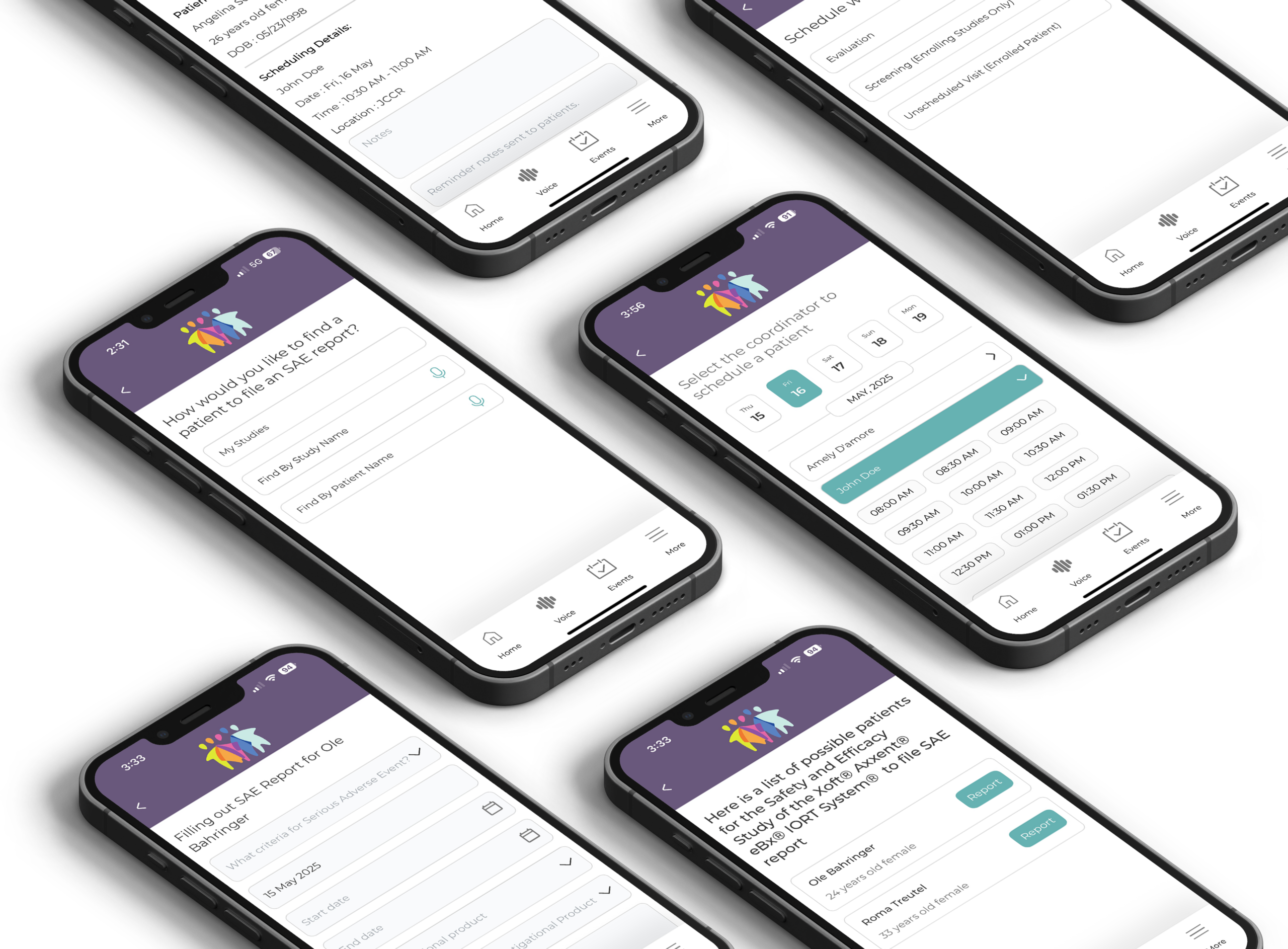
Screen and randomize patients while tracking visits and procedures performed.
Access and store trial data anywhere you have access to the internet.


System-generated appointment reminder texts to decrease missed visits and optimize retention.
Whether you’re running one study or managing multiple sites, Clinasyst scales with your needs.

Designed with built-in tools to ensure adherence to GCP, FDA, and other regulatory requirements.
Made to quickly be added across multiple trials.

Easy to use scheduling system with options to monitor visits and create team meetings with built-in reminders.

From a searchable patient database and financial tracking to tracking advertising campaigns and scalable solutions, discover how ClinAsyst can optimize your clinical trial workflow.
Be the first to know about new features, platform enhancements, and upcoming releases. Sign up for updates from the ClinAsyst team and get a front-row seat to what’s next in clinical trial innovation.

ClinAsyst offers affordable, scalable pricing so you only pay for the tools your site actually needs—no bloated costs or hidden fees.

Track invoicing, milestones, and payments in real time. ClinAsyst helps prevent missed billing and underreported work from cutting into margins.

HIPAA- and 21 CFR Part 11-compliant with U.S.-based hosting, role-based access, and audit trails to simplify inspections and protect data.
Got Questions? We have answers. Explore our FAQs to find quick solutions and insights about ClinAsyst.
ClinAsyst is an advanced clinical trial management platform designed to streamline patient management, financial tracking, and compliance for clinical research organizations of all sizes.
Yes, ClinAsyst is fully HIPAA-compliant, ensuring the highest standards of data privacy and security.
Absolutely. ClinAsyst offers seamless integration with popular EHR platforms, enabling uninterrupted workflows.
Yes, ClinAsyst is scalable and customizable, making it suitable for both small research sites and large organizations.
Implementation typically takes 2–4 weeks, depending on the complexity of your organization’s needs.
Of course! You can schedule a personalized demo through our website to see how ClinAsyst works for your specific requirements.
We offer 24/7 customer support via email and phone, along with an extensive resource hub for training and troubleshooting.
ClinAsyst has built-in tools to ensure adherence to industry regulations like HIPAA, GDPR, and FDA guidelines, keeping your trials audit-ready at all times.
Yes, we provide onboarding sessions and training resources to help your team get the most out of ClinAsyst.
ClinAsyst offers flexible subscription plans tailored to the size and needs of your organization. Contact us for detailed pricing or a custom quote.
Copyright © 2025 Clinasyst. All Rights Reserved.